Mass is a property of mater. The mass of an object is the same everywhere .mass can never be zero .mass does not change according to location . mass is scalar quantity .
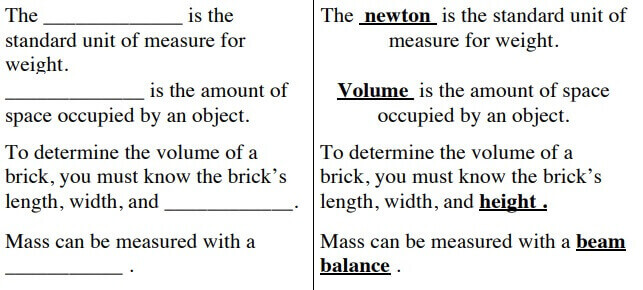
Anything that is mass-based and occupies space. Mass refers to the amount of weight an object has. The scale measures mass. Volume refers to the amount of space that matter occupies. It can be determined by multiplying the object’s length, width, and height or by using measuring cups and seeing how the water’s measurement changes inside the cup. Density is the quantity of matter that an object can hold within a specific space. To determine density, you need to divide the mass of the object’s volume by the object. D=M/V. A property is a thing that defines material things. Physical properties are the things you can identify about objects using your senses and without altering the object’s physical form. For instance that the shape of a ball is circular. Chemical properties are the things you can identify about an object by changing it into something completely new that can’t be reversed.
Examples: Wood can burn. The three types of matter are liquid, solid, and gas. Solids have a distinct shape and volume. It has significant importance, but it has no specific form. Gases are not actual size and have no body. An element is any substance comprised of one type of matter. Examples of such features include iron, gold, and oxygen. The atom is the smallest element in the universe which still retains the element’s characteristics. Chemical symbols are one – or two-letter words used to represent the component’s name. Example: O is for oxygen. Fe means Iron H stands for hydrogen, Hydrogen stands for hydrogen, and Au is for gold. Compounds are composed of two or more components joined together and can’t be separated. Example: Water is collected of oxygen, and hydrogen salt is salt and chloride. A molecule is the smallest element of a compound that contains the characteristics of the mix. Chemical formulas are an array of symbols that indicate the types and numbers of atoms that make up a chemical molecule.
Examples include: H2O is water while CO2 refers to carbon dioxide. Physical changes refer to an alteration in size, shape, or state of matter in which nothing new is created. Examples: tearing up a sheet of paper. A mixture is comprised of several substances that are easily separated. Examples: salad. Energy is the power to change. The term “solution” refers to a mix where particles of various substances are mixed equally across. For instance, Kool-Aid is a type of solution. Chemical changes lead to some or all new types of matter being formed. Examples: oxygen and iron are combined to form rust. Standard units are what that we measure using, like meters or liters and grams. Solvents are the component that dissolves the other leg. For instance, the Kool-Aid’s water is an example of a solvent. Solutes are the components of a solution that dissolves. For example, the powder found in Kool-Aid is a solute.