Sulfur is a chemical element bearing an atomic symbol S. The atomic number 16 is the tenth and fifth most common element throughout the Universe and earth and on the planet, respectively. At average temperatures and pressures, sulfur atoms make up cyclic Octatomic molecules (chemical formula of S8).
Sulfur is an essential component in both the perspectives of chemical and biological. It is a vital part of every living cell and is a significant element in enzymes, proteins, amino acids, vitamins, and cysteine, demonstrating its importance in biology.
Elemental sulfur is the basis for an 85% component in creating the most potent compound, H2SO4. Not only that but numerous significant compounds such as SO2 and H2S are produced using elemental sulfur, demonstrating its chemical significance.
How can I find the valence electron of sulfur(S)?
We know how to determine the valence electron for sulfur(S) quickly. The valence electron needs to be identified using several steps, and electron configurations are just one of the steps. It is impossible to locate the electron’s valence without knowing its composition.
If you know the electron configuration, the correct manner is a breeze to find the valence electrons for all elements. An article on this website explains the electron configuration, and you can look it up if you wish. This article, however, only examines electron configuration.
Science researcher Niels Bohr was among the first to present an understanding of the orbit of an atom. He created a model of the atom in 1913. The entire concept of the orbit was described in 1913. The electrons in the atom rotate around the nucleus in an equilateral direction. These circular paths are known as orbits(shells). The orbits(shell) are expressed as the number of. [ n = 1,2 3 4 . . .]
K is the title given to K, the name of the orbit(shell), L is the second, M is the third, and N is the designation of the 4th orbit(shell). the electron’s holding capability of each orbit is 2n2. [Where 1 = 1,2 + 3,4. . .]
Additionally to this technique, electron configuration can also be accomplished through sub-orbits. The German scientist Aufbau first suggested the idea of electron configuration using sub-orbits, and the Aufbau method is to perform electron configuration at lower energy levels.
Sub-orbitals are represented by the letter ‘l’. The Aufbau principle is that electrons in the atom will initially complete the orbital with the lowest energy and gradually complete the orbital with a higher power. These orbitals are called s and p, and they are also known as d, d and f. Their electron-holding capacity is 2 6 for p, ten and f = 14.
How many protons and electrons do sulfur(S) contain?
The nucleus is situated in the middle of the atom, and protons and neutrons are both located within the heart. The atomic number for sulfur(S) has been determined to be 16, and the Atomic number is the count of protons.
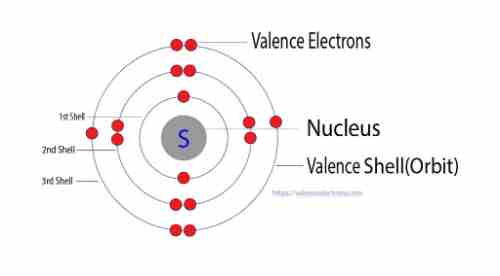
The number of protons within sulfur(S) is 16. Protons and electrons are found within a circular shell located just outside the nucleus, and this means that the sulfur atom contains an array of 16 electrons.
Explanation
Sulfur contains six electrons of valence.
Valence electrons are the most outermost electrons, and, consequently, they are at the most energy levels. This means that they are the electrons used in chemical bonding.
To find the number of electrons, look at the arrangement of electrons which shows the number of electrons in the different energy levels and orbitals.
The difference between Valence Electrons and Valency
The emitting electrons represent the total number of electrons present in the outermost shell of the atom (i.e. in the outermost orbital). The valence electrons of neutral atoms are sure; they can’t be changed (more than or lesser) at any time for any particular fraction, and it may or might not be identical to its value.
Valency is the number of electrons an atom could gain, lose, or share in the course of bond formation to achieve a stable electronic configuration, i.e. to complete an Octet. The valency of an atom could be different in different chemical reactions or compounds because of the various created bonds. Most of the time, valency alters or changes due to changes in the oxidation and reduction states.