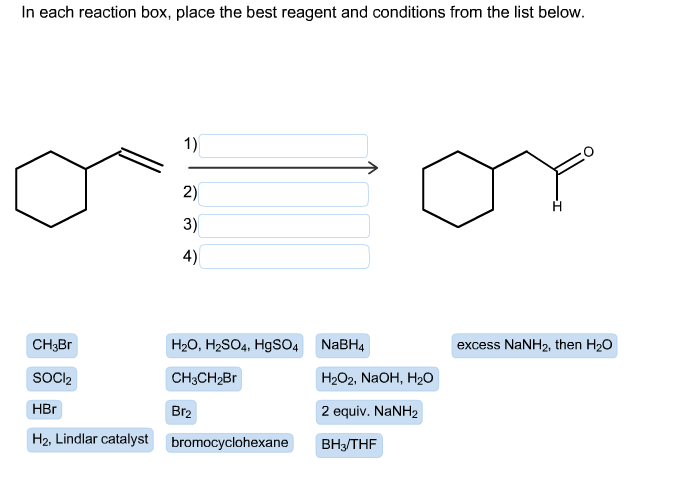
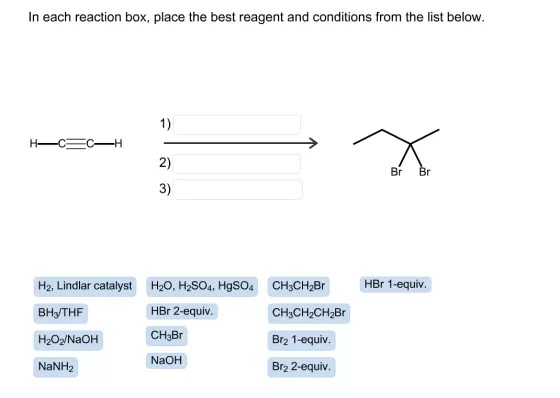
The article below will discover how to tackle reactions box problems to select the most suitable reagent and the right conditions. In essence, solutions to these issues are straightforward due to massive similarities and repeating patterns, but we must be extremely cautious due to the diverse requirements and structure.
What is the most important scientific idea you require to know to resolve this issue?
Our teachers have advised that you’ll have to apply an Addition Retrosynthesis concept to resolve this issue. If you need further Addition Practice with Retrosynthesis, you may also do Addition Retrosynthesis problems.
How to Solve “Place the best Reagent in Every Reaction Box” Issues
These are just a few of the main actions you must follow in solving your reaction box issues:
Step 1: Analyzing the reaction
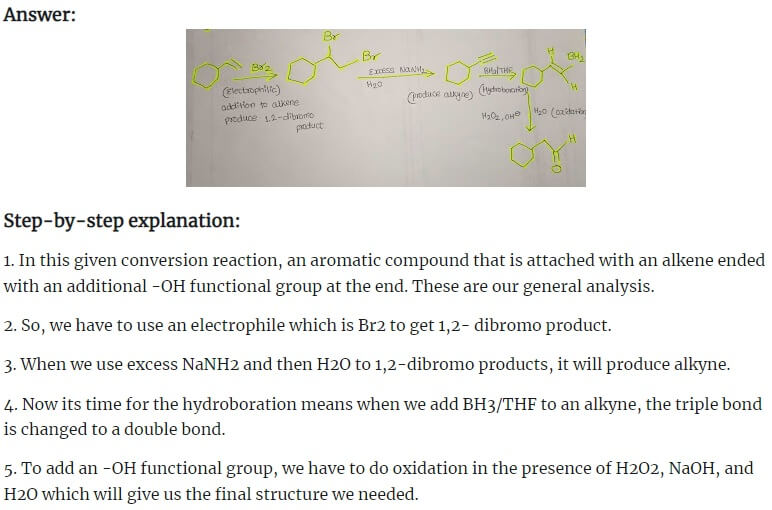
Answer
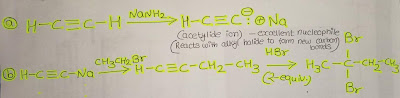
Step-by-step explanation:
- On the left, the structure is two carbons with triple bonds that mean it’s an alkyne known as acetylene or ethyne. On the other side, it has four carbons with one bond with two bromine atoms. Connected by the same carbon atom whose IUPAC is called 2,2-dibromobutane.
- The number of carbons increases, transforming the triple bond into single bonds. First, we need to utilize sodium amide (NaNH2), which gives us an acetylide Ion, a fantastic nucleophile. It can readily react with alkyl halides to create a new carbon bond.
- Our final product contains four carbons, which means we need to add two carbons, and CH3-CH2-Br is our only alternative. We will obtain 1-butyne; however, the final product does not have triple bonds and has two bromines attached to one atom.
- We now want to include two bromine groups and reduce triple bonds to single bonds. To do this, we need to have two equivalents to the HBr (i.e. Br2 2-Equiv.). In this procedure, the first bromine decreases the triple bond to double bond, and the second lower the bond from double to one. We will then be able to achieve our final product.
Example 2: (Four boxes total)