Activation energy is frequently examined in the field of physical chemistry, and it’s a crucial notion in the field of chemical kinetics and JEE. The concept of Activation Energy was coined by a Swedish scientist, Svante Arrhenius in 1889. Nowadays, we are taught about this idea since it aids us in understanding the power requirements required to conduct a chemical reaction and gives us control over our actions as well as our environment.
Most chemical reactions need some form of activation energy to proceed. It is therefore crucial that students know what energy activation is. This lesson will provide more information on this idea in the next lesson.
Why Is Activation Energy Needed?
If you mix two substances just a few collisions are likely to happen between the molecules reacting for the production of the products. This is the case especially if the molecules possess lower energy of kinetic. Thus, before a large percentage of reactants are transformed into products, the free energy in the system needs to be controlled. This energy is used to activate a reaction the slight boost it needs to begin. All exothermic reactions require activation energy to start. Like, for instance, a stack of wood will not begin combustion on its own. A match that is lit can give the energy needed to activate the combustion process. When the chemical reaction begins the release of heat from the reaction gives the energy needed to turn the reactant into the product.
A chemical reaction can happen without requiring any more energy. This is because the energy needed to initiate the reaction is normally provided by heat generated from the temperature of the surrounding environment. The heat increases the speed of molecules in the reaction increasing the chances of collisions as well as increasing the force of collisions. This increases the likelihood that bonds between reactants rupture, leading to the creation of new products.
Catalysts and Activation Energy
An ingredient that decreases the energy required to activate the chemical reaction is known as the catalyst. The way a catalyst works is a way to alter the state of transition of a chemical reaction. Catalysts do not consume the chemical reaction and do not alter the equilibrium value of the chemical reaction.
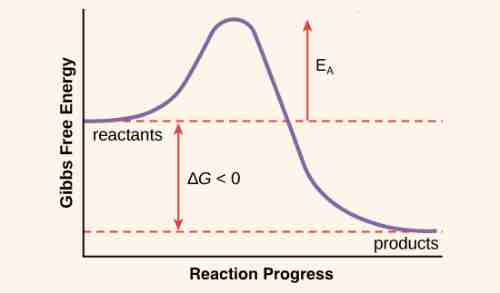
Relationship Between Activation Energy and Gibbs Energy
The term “activation energy” is used within the Arrhenius equation that calculates the energy needed to get over the transition phase of reactants into products. It is also known as the Eyring equation is an additional formula that is used to describe the rate of a reaction. However, instead of activation energy, it incorporates the Gibbs energy in the change state. The Gibbs Energy of the transition state is a factor in both the enthalpy as well as the entropy of the reaction. The energy of activation and Gibbs are both related but they are not mutually exclusive.
Activation energy
What is the reason why a reaction that releases energy that has negative G requires energy to continue? To understand why it is necessary to examine what happens to molecules in the reactant when they undergo the course of a chemical reaction. For the chemical reaction to happen it is necessary for some or all of the chemical bonds within the reactants to be broken so that new bonds, like those in those of the product, will form. To bring the bonds to the state where they can split, molecules must be bent (deformed bent or deformed) to create an unstable state, known as”the transition state. This is one of the most energy-efficient states, and a certain amount of energy, called activation energy has to be added for the molecule to attain it. Since the state of transition is in a state of instability, the reactants do not stay for long they quickly move on to the next phase in the process of chemical reactions.
The relationship between Gibbs’ energy and activation
Main article: Theory of Transition State
In the equation of Arrhenius, the expression activation energy (Ea) is utilized to define the amount of amount of energy needed to achieve the state of transition and the exponential relation to k = A exp(-Ea/RT) applies. The theory of transition states is an advanced model of the interaction between the rate of reaction and the change state. In the case of transition states, a similar mathematical relation, called the Eyring equation, is utilized to define the constant rate of a chemical reaction. k = (kBT + (h) exp(-DG+ / the rate). But instead of describing the dependence on temperature of the rate of reaction phenomenologically Eyring equation is a model of the basic steps of a process. In a multi-step procedure, there’s no clear connection between the two equations. However, the functional models for both the Arrhenius as well as Eyring equations have a lot in common, in a process that is one step there simple and meaningful chemical relationships could be established between Arrhenius as well as Eyring parameters.
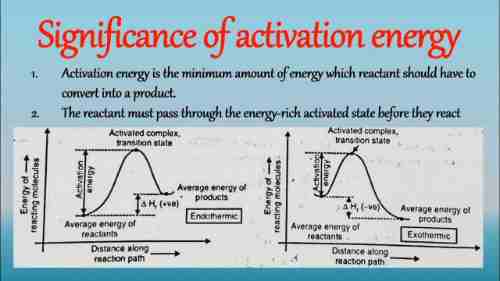
Instead of utilizing Ea, the Eyring equation employs the idea of Gibbs energy as well as its symbol DG+ to represent the Gibbs activation energy to reach the state of transition. The equation uses kB as well as h to represent both the Boltzmann as well as the Planck constants as well as Planck constants. Though the equations are like they are similar, it’s crucial to remember that the Gibbs energy has an entropic component as well as an enthalpic component. The Arrhenius equation shows this entropic element is taken care of by the pre-exponential coefficient A. Furthermore, we could define the Gibbs-free energy of activation as a function of the enthalpy as well as the entropy of activation. DG+ = DH+ T DS+. In the case of a unimolecular one-step reaction approximations for Ea = DH+ + RT, and A = (kBT/h) exp(1 + the DS++/R) remain valid. Be aware of the fact that, in Arrhenius’s theory itself, A is independent of temperature but here, it is a linear relationship with the temperature. If you are dealing with an unimolecular one-step reaction whose half-life at temperatures of room is approximately two minutes, DG+ corresponds to around 23 Kilocalories per mo. It is also the amount of Ea for a reaction that takes place over some time at temperatures of room temperature. Because of the rather tiny size of TDS+ and RT for normal temperatures of many reactions, when used in uninformed discussion, the terms Ea, DGplus, as well as DH+, are commonly confused and referred to as “activation energy”.
Entropy, enthalpy, and Gibbs energy for activation are better described in the form D++Ho, D++So, and D++Go. In each case, the”o” indicates that the quantity is evaluated against the standard state. Some writers do not include the word “o” to make it easier to understand the form of the notation. [13][14]
The free energy total change of the reaction is not dependent on the energy that activates it. Chemical and physical reactions may be exergonic or endergonic. However, the activation energy has no relation to the speed of a reaction. The total energy shift of a reaction is not affected by activation energy.
Negative activation energy
In certain instances, the rates of reactions decrease as they increase temperatures. In the case of an approximative relationship, the constant of rate is still able to be derived from an Arrhenius formula, and this will result in a negative number of Ea.
Reactions that exhibit negative activation energies are generally non-barrierless reactions, where the process relies on the capture of molecules that are in the potential well. The higher temperature results in an increase in the probability of collisions capturing each other (with greater glancing collisions, but not triggering a reaction since more momentum can carry collision particles away from the well) This is represented as an increase in the size of the reaction’s cross-section that shrinks with temperature. The situation does not lead to literal interpretations such as the potential height of a barrier. [15]
Multistep reactions include evident negative activation energy. In this case, the total rate constant k of a reaction with two steps A = B, B, and C can be calculated as K2K1 = k2 where K2 is the constant of the slow, rate-limiting second step, and K1 represents the equilibrium value for the first, rapid step. In certain reactions, K1 decreases in temperature faster than k2 does and k reduces as temperature increases, which corresponds to an observed negative activation energy. [16][17][18]
activation energy
Symbol Ea. Minimum energy needed for a chemical reaction to occur. When a chemical reaction occurs, molecules that are reacting come together, and chemical bonds are stretched out, fractured, and then formed when making the final products. In this reaction, the energy in the system rises by a significant amount, but after which it decreases to that of the product. The activation energy represents what is the difference between maximum energy and the energies of the reactants, i.e. it’s an energy barrier that must be negotiated for the reaction to continue. The activation energy is the determining factor in how much the rate of reaction is affected by the temperature. It is common to describe the activation energy in the joules per mole in the reaction mixture …. …
How to Use a Graph to Find Activation Energy
Another approach to estimate the energy involved in activating an action is to plot the ln k (the frequency constant) against the ratio 1/T (the opposite of temperature measured in Kelvin). The plot should form an unbroken line, which is expressed using the formula:
m = -Ea/R
with m being how steep the curve is. Ea is the energy for activation while R represents the gas constant at 8.314 J/mol-K. If you recorded temperature data either in Celsius or Fahrenheit make sure that you convert them to Kelvin before you calculate 1/T and make the graph.
If you could draw a graph of the energy produced by the reaction against the response coordinate, the difference in energies of the reactants as well as the product is DH The excess energy (the area of the graph higher than that of the products) is the activation energy.
Be aware that, although the majority of reactions increase in rate as temperatures increase There are some instances that the rate of reactions declines when temperature is raised. The negative reactions activate energy. Thus, although you must think that activation energy will be positive be aware that there is a possibility that it can even be negative.
Who Discovered Activation Energy?
Swedish scientist Svante Arrhenius proposed the term “activation energy” in 1880 to identify the minimal energy required for a group of chemical reaction substances to interrelate to form the products. In diagrams, activation energy can be represented as the highest point of the energy barrier that is between two minimal points of energy potential. The points that are minimums represent the energy levels of stable reactions and the products.
Exothermic reactions too, such as the burning of a candle need energy input. When it comes to the combustion process, an ignited match or extreme heat triggers the process. The energy generated by the process provides power to allow it to continue self-sustaining.